Hentet: https://pmc.ncbi.nlm.nih.gov/articles/PMC9249248/
. 2022 Jun 1;14(6):e25573. doi: 10.7759/cureus.25573
Abstract
The ongoing coronavirus disease 2019 (COVID-19) pandemic has turned into one of the most serious public health crises of the last few decades. Although the disease can result in diverse and multiorgan pathologies, very few studies have addressed the postmortem pathological findings of COVID-19 cases. Active autopsy findings amid this pandemic could be an essential tool for diagnosis, surveillance, and research. We aimed to provide a comprehensive picture of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) histopathological features of different body organs through a systematic review of the published literature. A systematic search of electronic databases (PubMed, ScienceDirect, Google Scholar, medRxiv, and bioRxiv) for journal articles of different study designs reporting postmortem pathological findings in COVID-19 cases was performed. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used for conducting the review. A total of 50 articles reporting 430 cases were included in our analysis. Postmortem pathological findings were reported for different body organs: pulmonary system (42 articles), cardiovascular system (23 articles), hepatobiliary system (22 articles), kidney (16 articles), spleen and lymph nodes (12 articles), and central nervous system (seven articles). In lung samples, diffuse alveolar damage (DAD) was the most commonly reported finding in 239 cases (84.4%). Myocardial hypertrophy (87 cases, 51.2%), arteriosclerosis (121 cases, 62%), and steatosis (118 cases, 59.3%) were the most commonly reported pathological findings in the heart, kidney, and the hepatobiliary system respectively. Autopsy examination as an investigation tool could lead to a better understanding of SARS-CoV-2 pathophysiology, diagnosis, and management, subsequently improving patient care.
Introduction and background
The novel coronavirus disease 2019 (COVID-19) pandemic has become one of the most challenging public health crises for decades. It first emerged in Wuhan, China, in late December 2019 and is believed to be caused by infection with the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) virus [1]. The first cases of COVID-19 in China were believed to be of zoonotic origin, but the global spread of the disease has been mainly travel-related. The disease has spread from China to nearly 200 countries worldwide [2]. The virus is easily transmissible via droplets and fomites or when bodily fluids of the infected individual come into contact with another person’s face, mouth, eyes, or nose [3].
Regarding the pathogenesis of COVID-19, angiotensin-converting enzyme 2 (ACE2), which is highly expressed in the respiratory tract, acts as a receptor to SARS-CoV-2. The virus invades the human cells, causing massive destruction and inflammation of different organs and subsequently affecting the vascular supply and even progressing to fibrosis [4]. The main clinical manifestations include fever, cough, fatigue, and shortness of breath. Other less common symptoms include headache, sore throat, and rhinorrhea. Also, one-fifth of patients (20%) presented with severe symptoms such as respiratory failure, multiorgan failure, and septic shock, all of which necessitate intensive care [5]. Although COVID-19 mainly affects the respiratory system, there have been reported cases of cardiogenic and renal involvement in patients without previously known heart or renal diseases [6,7].
The case-fatality rate for COVID-19 is variable across different nations and ranges from 11.75% in Italy to 0.37% in South Africa. The mean recovery time is two weeks for mild cases and three to six weeks for severe or critical cases [8]. The diagnosis of COVID-19 relies mainly on reverse-transcription polymerase chain reaction (RT-PCR) with some emerging evidence endorsing the utility of characteristic CT and laboratory findings [9]. COVID-19 is a member of the coronavirus family, which includes Middle East Respiratory Syndrome-related coronavirus (MERS-CoV) and SARS-CoV [10]. Both MERS-CoV and SARS-CoV are believed to affect humans and cause interstitial pneumonia, pneumocyte hyperplasia, and acute diffuse alveolar damage (DAD) [11,12]. The diverse histopathological findings associated with COVID-19 infections suggest that multiple organs are affected by the virus, with the pulmonary system being the most common system to be affected. Carsana et al. showed in their study the wide variety of pathological findings related to COVID-19 in the respiratory system. They found that pneumocytes desquamation, pulmonary edema, and DAD are the most common microscopic findings [13].
As of August 10, 2020, the number of COVID-19 cases worldwide has surpassed 20,162,474 million, with almost 737,417 deaths. However, the number of studies addressing the postmortem autopsy findings of COVID-19 patients is still scarce given the number of deaths. This could be attributed to the fears of contagion associated with COVID-19 infection. Since the beginning of the pandemic, the Centers for Disease Control and Prevention (CDC) have released interim guidelines for the collection and analysis of clinical specimens that might contain SARS-CoV-2 [14].
Active autopsy findings amid emerging epidemic diseases have been identified as an essential tool for diagnosis, surveillance, and research. Pathologists are usually among the first healthcare professionals to identify novel infectious agent outbreaks [15]. Our aim in this systematic review is to provide a comprehensive picture of the SARS-CoV-2 histopathological features of different body organs in postmortem autopsies through a systematic search of the published literature. We believe that this will foster a better understanding of the mechanisms of injury and pathophysiology of severe SARS-CoV-2 infection and subsequently improve patient care.
Review
Methods
This study followed the recommendations established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Appendix 1) [16].
Sources of Information
A predetermined protocol was used to perform this systematic review by using the following databases: PubMed, Google Scholar, ScienceDirect, and medRxiv. The reference lists of relevant studies were manually searched to identify cited articles that were not captured by electronic search.
Selection Criteria
Articles were included if they met the following eligibility criteria: (1) addressed pathological reports of COVID-19 autopsies or postmortem cases, (2) involved human subjects (at least one case), (3) all study designs were involved (case report, case series, cross-sectional, case-control, randomized and non-randomized studies), and (4) no language restrictions were applied.
Study Selection and Search Terms
The search terms and keywords across the different databases have been provided in Appendix 2. The selection was broad enough to include as many studies as possible. In the initial phase, two independent reviewers (H.H. and A.B.) screened the titles and abstracts of the articles by using the Rayyan QCRI® website [17]. As a result, all non-relevant articles were excluded. In the second phase, the full texts of the remaining articles were independently reviewed for the final selection of eligible studies. Any disagreement between the two reviewers was resolved by a third reviewer (T.B.).
Quality Assessment and Risk of Bias
To assess the internal validity of the included studies, we used different tools according to the study design. For cross-sectional studies, the Newcastle-Ottawa Quality Assessment Scale (NOS) (modified for cross-sectional studies) was used after removing items that relate to comparability and adjustment. The tool contains three major subsections (Selection, Comparability, and Outcome). A score for quality, modified from the tool, was used to assess the appropriateness of study design, recruitment strategy, sample representativeness, reliability of the outcome, sample size provided, and appropriate statistical analyses. At least two reviewers (H.H., A.B., T.B.) independently ranked these domains. When the independent evaluations of the ranks differed between the two reviewers, they discussed disagreements to reach a consensus. For case reports and case series, a version of the NOS checklist has been adapted by Murad et al. to assess the methodological quality of case reports and case series [18]. By this approach, we assessed the quality of each study with regard to four domains: selection, ascertainment, causality, and reporting. From the results of each checklist, if 25% or less of the criteria were addressed, the article was scored as poor; if 26-50% of the criteria were addressed, the article was scored as fair; if 51-75% of criteria were addressed, the article was scored as good; and if 76-100% of the criteria were addressed, the article was scored as excellent.
Data Extraction
A single author (A.B.) extracted the variables from each included study. The data from the final list of included articles were transferred onto an online Google Sheet. Several study characteristics were extracted, including:
General characteristics such as study type, country of origin, article language, and sample size.
Study population demographics like age and gender.
Clinical findings like symptomatology, lab findings, and patient comorbidities.
Histopathologic and microscopic findings of different organs.
Results
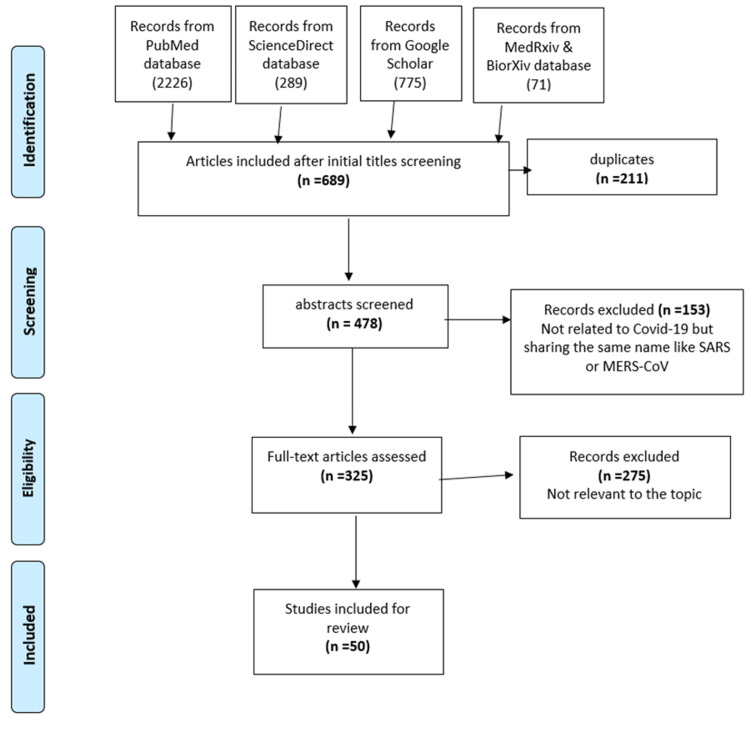
Figure 1 shows a flow chart illustrating the procedure for the selection of studies. Initially, we identified 3,297 studies from five databases as follows; PubMed (2,262), ScienceDirect (189), medRxiv and bioRxiv (71), and Google Scholar (775). After the initial title screening, we were left with 689 articles; 211 duplicates were removed, leaving 478 for abstract screening. After excluding non-relevant articles, 50 articles were available for the final analysis.
Figure 1. Flow chart showing the procedure for the selection of studies.
Characteristics of Included Studies
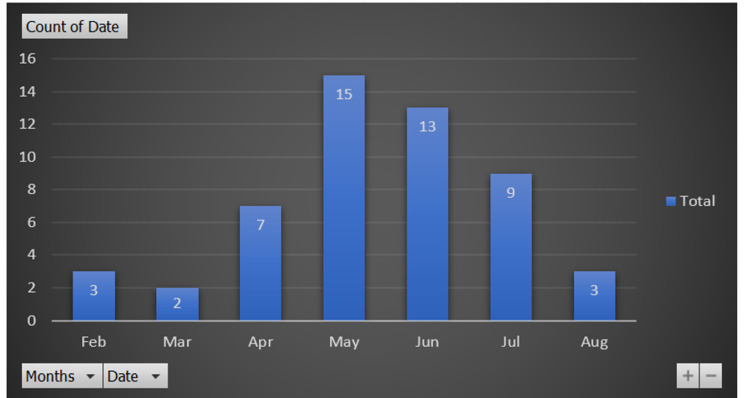
A total number of 50 studies were included in our systematic review, with 430 cases overall. Regarding the date of publication, only three studies were published in February, two in March, and seven in April, while 15 were published in May, which is the month with the highest number of publications (Figure 2). Regarding the type of published articles, case reports and case series were the most common categories (39 studies), followed by cross-sectional studies (10 studies), and there was one cohort study. Regarding the country of origin of the published studies, there were about 16 countries. The USA was the country with the most publications (16 studies), followed by China (10 studies), Germany (six studies), Italy (five studies), Switzerland (two studies), and there was one study from each of the following countries: Iran, Finland, Austria, Belgium, Japan, Spain, Netherlands, UK, Romania, Austria, and Denmark. As for the language of the published articles, only English and Chinese were represented: 47 studies were in English while three were in Chinese (Table 1).
Table 1. General characteristics of the included studies.
| Authors | Country | Language | Design | Number of cases | Study quality |
| Yao et al. [49] | China | Chinese | Case report | 3 | Excellent |
| Barton et al. [24] | USA | English | Case report | 2 | Good |
| Xu et al. [25] | China | English | Case report | 1 | Good |
| Tian et al. [27] | China | English | Case series | 4 | Good |
| Tian et al. [26] | China | English | Case report | 2 | Good |
| Su et al. [28] | China | English | Cross-sectional study | 26 | Good |
| Carsana et al. [13] | Italy | English | Cross-sectional study | 38 | Good |
| Schaller et al. [20] | Germany | English | Cross-sectional study | 10 | Fair |
| Menter et al. [50] | Switzerland | English | Cross-sectional study | 21 | Excellent |
| Edler et al. [51] | Germany | English | Cross-sectional study | 12 | Excellent |
| Remmelink et al. [52] | Belgium | English | Cross-sectional study | 17 | Excellent |
| Buja et al. [29] | USA | English | Case series | 3 | Good |
| Bradley et al. [30] | USA | English | Case series | 14 | Good |
| Lax et al. [53] | Austria | English | Cross-sectional study | 11 | Excellent |
| Wichmann et al. [31] | Germany | English | Cohort study | 12 | Good |
| Rapkiewicz et al. [21] | USA | English | Case series | 7 | Fair |
| Martines et al. [32] | USA | English | Case series | 8 | Good |
| Magro et al. [54] | USA | English | Case series | 5 | Excellent |
| Fox et al. [33] | USA | English | Case series | 10 | Good |
| Bryce et al. [55] | USA | English | Case series | 25 | Excellent |
| Prilutskiy et al. [34] | USA | English | Case series | 4 | Good |
| Konopka et al. [22] | USA | English | Case report | 1 | Fair |
| Fitzek et al. [56] | Germany | English | Case report | 1 | Excellent |
| Zhang et al. [35] | China | English | Case report | 1 | Good |
| Li et al. [36] | USA | English | Case report | 1 | Good |
| Cipolloni et al. [37] | Italy | English | Case report | 2 | Good |
| Adachi et al. [57] | Japan | English | Case report | 1 | Excellent |
| Flikweert et al. [58] | Netherlands | English | Case series | 7 | Excellent |
| Grillo et al. [38] | Italy | English | Case series | 8 | Good |
| Xu et al. [39] | China | Chinese | Case series | 10 | Good |
| Wu et al. [40] | China | Chinese | Case series | 10 | Good |
| Youd et al. [41] | UK | English | Case series | 9 | Good |
| Konopka et al. [23] | USA | English | Case series | 8 | Fair |
| Schaefer et al. [60] | USA | English | Case series | 7 | Excellent |
| Ackermann et al. [59] | USA | English | Case series | 7 | Excellent |
| Bösmüller et al. [61] | Germany | English | Case series | 4 | Excellent |
| Suess et al. [42] | Switzerland | English | Case report | 1 | Good |
| Sonzogni et al. [62] | Italy | English | Cross-sectional study | 48 | Excellent |
| Wang et al. [63] | China | English | Cross-sectional study | 2 | Excellent |
| Bruni et al. [43] | Italy | English | Case report | 1 | Good |
| Colmenero et al. [44] | Spain | English | Case series | 7 | Good |
| Beigmohammadi et al. [45] | Iran | English | Case series | 7 | Good |
| Heinrich et al. [46] | Germany | English | Case report | 1 | Good |
| Wang et al. [47] | China | English | Case report | 2 | Good |
| Ducloyer et al. [64] | France | English | Case report | 1 | Excellent |
| Kantonen et al. [65] | Finland | English | Case series | 3 | Excellent |
| Reichard et al. [48] | USA | English | Case report | 1 | Good |
| Cîrstea et al. [66] | Romania | English | Case report | 1 | Excellent |
| Schwensen et al. [67] | Denmark | English | Case report | 1 | Excellent |
| Santoriello et al. [68] | USA | English | Cross-sectional study | 42 | Excellent |
Figure 2. Timeline distribution of published articles .
Quality of Evidence
We used the GRADE framework for judging the precision and confidence estimate in the review. Generally speaking, the evidence derived from observational studies was classified as low quality [19]. Regarding the risk of bias assessment in the review, four articles scored between 26 and 50%, which is considered “Fair” [20–23]; 26 articles scored between 51 and 75%, which is considered “Good” [13,24–48], and 20 articles scored more than 76%, which is considered “Excellent” [49–68]. A high degree of inconsistency was noticed in the review as the study populations were heterogeneous with respect to main characteristics like age, gender, and comorbidities. Although there were no language restrictions applied in the review, publication bias may appear due to the fact that the number of published articles was small, especially at the beginning of the pandemic. Moreover, a very small number of countries were reporting autopsy findings. Regarding the indirectness, the majority of included studies used the same tool in diagnosing COVID-19, which is RT-PCR, the same tool in identifying histopathological findings, and the studied population varied between studies. Hence, the quality of evidence was rated as “Moderate” (Appendix 4).
Clinical Findings of the Cases
The review described a total of 430 patients with COVID-19. Among the included patients, gender was reported in 349 [297 males (85.1%) and 133 (14.9%) females]. The ages of the patients for whom age was reported ranged from 11 to 94 years. Regarding the presenting symptoms of patients whose clinical symptoms were reported (192 patients), fever was reported in 121 patients, followed by cough in 103 patients, and dyspnea in 91 patients [20–27,29–33,35,37,39–43,45–49,52–54,56–58,60,61,63–67]. Regarding the pre-existing comorbidities in patients whose medical history was reported, hypertension was found in 210 patients (48.8%), followed by coronary heart disease in 190 (44%), diabetes in 134 patients (31%), chronic kidney disease in 96 patients (22.3%), obesity in 64 patients (14.8%), chronic lung disease in 54 patients (12.5%), and cancer in 50 patients (11.6%) [13,20,21,23–33,35,39–42,45–66,68]. Regarding the organs included, this review observed the reported histopathology of different organs as follows: lungs and the pulmonary system were the most commonly described organs (42 articles) [13,20–27,29–33,35–38,40–42,45–47,49–61,63–67], followed by the heart in 23 articles [20,21,24,25,27,29,30,32,33,41,42,45–47,49–53,55,64,66,67], liver in 21 articles [20,21,24,25,27,29,30,32,42,45,46,49–53,55,62–64,66], kidneys in 16 articles [21,24,28–30,32,47,49–53,55,57,66,68], spleen and lymph nodes in 12 articles [21,29,30,32,34,39,50,51,53,55,57], central nervous system (CNS) in seven articles [20,30,46,48,52,55,65], skin in two articles [44,54], gall bladder in one article [43], and pharynx in one article [51].
Laboratory Investigations
In all of the included studies, RT-PCR on the nasopharyngeal swab was the predominant method used to confirm the positivity of COVID-19. RT-PCR on endotracheal aspirate swab was reported in one patient [67]. RT-PCR on skin biopsy was reported in seven patients [44]. Chest imaging, whether CT or X-ray, was reported in 206 patients (47.9%) [13,20,22,24–33,35,40,45,47,48,51,53,54,56–60,63,64,67]. Postmortem RT-PCR of the lung parenchyma was reported in 91 cases (21%) [23,24,30,32,35,37,40,41,46,47,51,52,58,64,66].
Lung Histopathological Findings
In the 42 articles that described lung pathology, the most commonly reported pathological findings were diffuse alveolar injury in 239 cases (84.4%) [13,20–27,29–33,35–38,40–42,45–47,49–52,55–61,63–67], followed by hyaline membrane formation in 184 cases (65%) [13,20–25,27,29,31,33,36,37,40–42,45–47,49–58,61,63,64,67], lymphocyte and/or monocyte infiltrates in 172 cases (60.7%) [13,20,21,24–27,29–31,33,35–37,40–42,45–47,49–56,58,61,64,66,67], pneumocyte hyperplasia in 171 cases (60.4%) [13,20,22,26,27,29,30,35,37,40–42,45–47,49,51–61,64,66], pulmonary microthrombi in 151 cases (53.3%) [13,21–23,29,31–33,36–38,46,49,51–53,55,56,58–61,65,66], fibrin exudation in 112 cases (39.8%) [13,21,22,29,33,35,37,40–42,45,47,49,51,52,54,56,58,61,63], lung fibrosis in 97 cases (34.2%) [13,20,26,30,31,33,35,38,49,51,52,56–58,63,67], intra-alveolar neutrophilic infiltration in 92 cases (32.5%) [13,20,22,24,27,29,31,33,37,40,42,47,49–51,54,58,61], intra-alveolar hemorrhage in 86 cases (30.4%) [13,27,29,30,32,33,42,45,47,49,50,52,54,57,66], interstitial thickening in 80 cases 28.3%) [13,20,26,27,30,32,35,37,41,49,56,59,61,66], vascular congestion in 77 cases (27.2%) [13,21,24,26,27,29,31,40,42,46,49,50,54,57], pneumocyte damage in 73 cases (25.8%) [13,21,25,27,33,35,47,49,59,61,63], squamous metaplasia in 68 cases (24%) [13,20,29,31,32,38,45,47,51,57,60,61,67], viral inclusion in 45 cases (15.9%) [13,26,30,32,49,55,61], serous exudation in 19 cases (6.7% [47,49,52,59], fibrinoid vascular necrosis in 17 cases (6%) [20,27,45,50,54,61], and pulmonary embolism in 14 cases (4.9%) [29,30,41,50,55,58] (Appendix 3).
Heart Histopathological Findings
In the 23 articles that described heart pathology, the most commonly reported pathology was myocardial hypertrophy in 87 cases (51.2%) [27,29,30,32,49,50,52,53,55], followed by myocardial fibrosis in 85 cases (50%) [27,29,30,32,33,41,46,47,51–53,55], coronary small vessel disease in 44 cases (25.9%) [21,24,29,41,50,53,55,64], myocardial cell infiltrate in 27 cases (15.9%) [20,21,25,45,49,51,53,55,66], cardiac amyloidosis in 10 cases (5.9%) [30,50,53], and myocardial necrosis in nine cases (5.3%) [45,47,49,50] (Appendix 3).
Liver Histopathological Findings
In the 21 articles that described liver pathology, the most frequently reported pathology was steatosis in 118 cases (59.3%) [21,24,25,27,29,30,32,34,42,45,46,50,52,53,55,62–64,66], followed by fibrosis in 62 cases (31.1%) [20,29,53,55,62,66], hepatic congestion in 59 cases (29.6%) [30,34,45,51–53,66], cellular infiltrate in 54 cases (27.1%) [20,25,27,30,53,62,63,66], hepatic necrosis in 44 cases (22.1%) [21,27,30,42,45,49,53,62,63], cholestasis in eight cases (4%) [53], and cirrhosis in four cases (2%) [27,52] (Appendix 3).
Kidney Histopathological Findings
In the 16 articles that described kidney pathology, the predominantly reported pathology was arteriosclerosis in 121 cases (62%) [24,28–30,51,55,68], followed by nephrosclerosis in 91 cases (46.7%) [24,30,55,68], acute tubular injury in 86 cases (44.1%) [21,28,32,49,50,53,55,66,68], glomerulosclerosis in 70 cases (35.9%) [28–30,32,47,52,53], tubular cast in 38 cases (19.5%) [21,28,30,49,52], glomerular fibrin thrombus in 21 cases (10.7%) [21,28–30,55,57,66,68], and viral particles in 16 cases (8.2%) [21,28,29] (Appendix 3).
Immune System (Spleen and Lymph Node) Histopathological Findings
In the 12 articles that described spleen pathology, the most commonly reported pathology was lymphocyte depletion in 38 cases (31.4%) [21,29,30,39,49,53,55], followed by hemophagocytosis in spleen in 12 cases (9.9%) [34,39,55,57]. In the 11 articles that described lymph node pathology, the most common pathology that had been reported was lymphocyte depletion in 23 cases (20.7%) [21,34,49,53,55], followed by hemophagocytosis in the lymph nodes in 22 cases (19.8%) [30,32,34,55,57].
CNS Histopathological Findings
In the seven articles that described CNS pathology, the most commonly reported pathology was cerebral hemorrhage in 11 cases (15.5%) [30,48,52,65], followed by focal spongiosis in 11 cases (15.5%) [48,52], vascular congestion in 11 cases (15.5%) [52,55], and diffuse or focal ischemic necrosis in nine cases (12.7%) [52,55].
Skin Histopathological Findings
In the two articles that described skin pathology, the most commonly reported pathology was thrombogenic vasculopathy in four cases (10, 0.4%) [44,54], followed by perivascular inflammation in two cases (10, 0.2%) [44,54], and vasculitis in one case [44].
Gall Bladder Histopathological Findings
In the one article that described gall bladder postmortem pathology, inflammatory infiltration and endoluminal obliteration of vessels with wall breakthrough, hemorrhagic infarction, and nerve hypertrophy were reported in a single case [43].
Pharynx Histopathological Findings
One study described pharyngeal postmortem pathology. The study included eight cases, seven of which reported mild to pronounced lymphocytic pharyngitis [51].
Discussion
This systematic review identified 50 studies with a total of 430 patients and postmortem pathological findings of different body organs. Since the beginning of the pandemic, the body of evidence and the number of published studies have increased over time, but it is still limited compared to the number of COVID‐19 deaths (almost one million deaths). There were only 16 countries that contributed to publishing autopsy reports of the COVID-19 deaths, which is considered very low given the fact that the disease affected nearly 200 countries all over the world [2]. With regard to the timeline of the published studies, it took us five months (up to May 2020) to find an appropriate number of publications addressing the postmortem pathological findings. One of the main reasons for the scarcity of published literature is the fear of COVID-19 infection transmission during postmortem examinations and the perceived risk among healthcare professionals, especially pathologists, about this «new» disease, coupled with a poor understanding of its pathological mechanism, especially at the beginning of the pandemic [69]. Moreover, in some countries, the number of safe autopsy rooms is very low, which, according to the WHO and CDC guidelines, is considered one of the barriers that contributed to the scarcity of evidence [70–72].
Postmortem Pulmonary Findings
Regarding the postmortem pulmonary pathology, our review showed that various histopathological findings had been identified among COVID-19 cases. Diffuse alveolar injury, hyaline membrane formation, pneumocyte hyperplasia, microthrombi, fibrin exudation, pulmonary fibrosis, and intra-alveolar hemorrhage are among the most frequently reported pathological findings. DAD has been the most frequently reported among all pulmonary findings with 239 cases (84%). On second thought, these pathological findings should not be seen as one of the COVID-19 attributes without considering other important factors affecting the course of illness like age, symptoms, comorbidities, and management plan. For example, Wichmann et al. investigated the possibility of attributed venous thromboembolism in a cohort of COVID-19 patients. Autopsy results found that deep venous thrombosis was found in 58% of the cases, and this has been linked to COVID-19 while ignoring the fact that the majority of patients suffered from atrial fibrillation, coronary heart disease, and cancers, which have been proven to be decisively determinant factors in developing thromboembolism [31].
On the other hand, similar pathological and autopsy findings have been reported in deceased patients with other coronavirus infections such as MERS-CoV and severe acute respiratory syndrome (SARS). NG et al. published a case report of a deceased 45-year-old patient with MERS-CoV with autopsy findings. Postmortem pulmonary findings included DAD, type 2 pneumocyte hyperplasia, interstitial infiltrate, alveolar fibrin deposits, and prominent hyaline membranes [73]. Alsaad et al. also reported DAD in their report that involved a 33-year-old patient with MERS-CoV [11]. Moreover, Franks et al. reported in their study of eight SARS patients that DAD was the main pathological finding. In contrast, Nicholls et al. reported other findings like the focal deposition of fibrin along the exposed basement membrane [12,74]. On the other hand and in non-coronaviruses pulmonary infections like H1N1 cases, histopathological findings such as septal inflammation, congestion, and thickening of alveolar septae, patchy peripheral hemorrhage, and diffuse alveolar hemorrhage have been reported in different studies [75–78].
Other Organ Findings
Regarding the postmortem cardiac pathology, there were 23 studies with a total number of 87 cases addressing the histopathological findings in the heart. Myocardial hypertrophy, small coronary vessels, cardiac fibrosis, cardiac cell infiltrates, and cardiac amyloidosis were the main findings. Although viral myocarditis has been reported in patients with SARS-CoV-2, lymphocyte infiltrate was found only in one case reported by Buja et al. during immunohistochemical (IHC) staining [29,79]. These pathological findings could be attributed to the comorbidities of affected patients, as most of them suffered from hypertension, diabetes, or coronary heart disease. On the other hand, myocardial edema and fibrosis have been recorded in deceased patients with SARS and MERS-CoV [73,80,81]. While the studies in this review reported that nephrosclerosis, arteriosclerosis, glomerulosclerosis, and acute tubular injury were the most commonly reported findings in the postmortem renal biopsies, other pathological findings like hyaline arteriolosclerosis, patchy interstitial inflammation, and granular casts have been reported in other coronavirus cases like SARS and MERS-CoV [73,82,83]. Regarding the pathological findings in the hepatobiliary system, our review found that hepatic fibrosis, steatosis, cirrhosis, and interstitial inflammations were the main findings. In contrast, other pathological findings were reported in patients with SARS-CoV-1 infection, such as lymphocytic infiltrate and balloon degeneration [84]. As for histopathological findings of the spleen and lymph nodes, lymphocyte depletion and hemophagocytosis of the spleen and lymph nodes were the main findings. Our results were consistent with similar pathological findings from other coronavirus infections [80,85].
Although SARS-CoV-2 has not been detected in the spinal fluid, our study suggests that COVID-19 is capable of infecting the CNS via olfactory and trigeminal nerves, thereby causing cerebral hemorrhage, focal spongiosis, and vascular congestion [86]. On the contrary, in the case of SARS infection, RT-PCR has detected the genomic sequences of the virus in cerebral spinal fluid and brain tissue specimens and was responsible for brain edema and neuronal degeneration [82,87].
Limitations of the study
As is typical of any research, we faced many limitations while conducting the review. Firstly, in this study, we focused on the available studies in certain databases in the first months of the pandemic, and hence government reports and other relevant gray literature were not included in this review; therefore, publication bias is a possibility. Second, due to the scarcity of evidence, we decided to include preprints. These publications had not yet undergone peer review. However, since we assessed the risk of bias in these studies, we feel that the benefits of including the data from these preprints in our review outweigh the risks. Third, we have included only 50 articles, but we cannot ignore the fact that the number of publications is increasing on a daily basis, and we might have missed the recently published ones. Fourth, missing information in some of the published articles has been a challenge. Many articles did not report the basic characteristics of the cases like gender, comorbidities, and clinical course of the disease.
Conclusions
Postmortem histopathological biopsies play an essential role in helping us understand the pathophysiology of SARS-CoV-2 infection. COVID-19 affects different body organs with different pathological features throughout the course of the infection. Cellular destruction, vascular invasion, and fibrous formation have been identified in the pulmonary, hepatobiliary, and renal systems. Further research is needed to gain a better understanding of the disease and to explore the extent of its effects on different organs and tissues.
Appendices
Appendix 4: quality checklist of selected studies
https://docs.google.com/spreadsheets/d/1YZZVEUz6uO8Iv9nlZ1OXNCLiux3LUeHbo9ioQFuBsJc/edit?usp=sharing
Appendix 3: microscopic and gross pictures
https://docs.google.com/spreadsheets/d/1d_yuTiLK5P6y18FwEqW57vs2wbEnA5zMz7WfC0dIS2M/edit?usp=sharing
Appendix 2: search strategy
Table 2. Appendix 2: search strategy.
| Database | Search limitation | Concept | Search term/strategy | |
| Mesh OR Keywords | ||||
| PubMed | Up to August 2020. Adult English search field: title, abstract, and full text | #1 | Coronavirus | (“COVID19” [Title/Abstract] OR “COVID-19” [Title/Abstract] OR “coronavirus disease 2019” [Title/Abstract] OR “2019 novel coronavirus infection” [Title/Abstract] OR “coronavirus disease-19” [Title/Abstract] OR “2019-nCoV disease” [Title/Abstract] OR “2019nCoV” [Title/Abstract] OR “2019 novel coronavirus disease” [Title/Abstract] OR “2019-nCoV infection” [Title/Abstract] OR “SARS-CoV-2” [Title/Abstract] OR “SARS2” [Title/Abstract] OR “Wuhan coronavirus» [Title/Abstract]) OR («COVID-19» [Supplementary Concept] OR «severe acute respiratory syndrome coronavirus 2» [Supplementary Concept]) |
| #2 | Postmortem pathology | «Autopsy»[Mesh] OR «Forensic Pathology»[Mesh] OR “autops*” [Title/Abstract] OR “postmortem” [Title/Abstract] OR “post-mortem” [Title/Abstract] OR “Histopath*” [Title/Abstract] OR “death” [Title/Abstract] OR “specimen” [Title/Abstract] OR “biopsy” [Title/Abstract] OR “cytopathology” [Title/Abstract] OR “immunopathology” [Title/Abstract] | ||
| ScienceDirect | (“2019-nCoV” OR “COVID-19” OR “novel coronavirus” OR “2019-nCoV infection” OR “SARS-CoV-2” OR “Wuhan coronavirus») AND (Autopsy OR «immunopathology» OR «autopsies» OR «specimen» OR «histopathology» OR «histopathological» OR «biopsy» OR «Cytopatho») | |||
| Google Scholar | (“2019-nCoV” OR “COVID-19” OR “novel coronavirus” OR “2019-nCoV infection” OR “SARS-CoV-2” OR “Wuhan coronavirus») («Autopsy» OR «Postmortem» OR pathology OR autopsies OR gravid OR histopathology OR specimen OR cytopathology OR immunopathology) | |||
| medRxiv and bioRxiv | Novel coronavirus AND postmortem pathology | |||
Appendix 1: PRISMA 2009 checklist used for the review
Table 3. Appendix 1: PRISMA 2009 checklist used for the review.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
| Section/topic | # | Checklist item | Reported on page # |
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | Page 1: (Title) |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | Page 2 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | Page 4 (Introduction) |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS) | Page 4 (at end of the Introduction) |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., web address), and, if available, provide registration information including registration number | A nonpublished protocol available upon request |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale | Page 5 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and the date last searched | Page 5 |
| Search | 8 | Present a full electronic search strategy for at least one database, including any limits used, such that it could be repeated | Appendix 1 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in the systematic review, and, if applicable, included in the meta-analysis) | Page 5 |
| Data collection process | 10 | Describe the method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | Pages 5, 6 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made | Page 6 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing the risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | Page 6 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means) | Pages 7-11 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis | NA |
Footnotes
The authors have declared that no competing interests exist.
References
- 1.A new coronavirus associated with human respiratory disease in China. Wu F, Zhao S, Yu B, et al. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rolling updates on coronavirus disease. [ Mar; 2022 ];Organization WH. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen 2020
- 3.Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Jayaweera M, Perera H, Gunawardana B, Manatunge J. Environ Res. 2020;188:109819. doi: 10.1016/j.envres.2020.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. Lukassen S, Chua RL, Trefzer T, et al. EMBO J. 2020;39:0. doi: 10.15252/embj.20105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. Li LQ, Huang T, Wang YQ, et al. J Med Virol. 2020;92:577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical characteristics of coronavirus disease (COVID-19) patients with gastrointestinal symptoms: a report of 164 cases. Zhang H, Liao YS, Gong J, Liu J, Xia X, Zhang H. Dig Liver Dis. 2020;52:1076–1079. doi: 10.1016/j.dld.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.At the heart of COVID-19. Khan IH, Zahra SA, Zaim S, Harky A. J Card Surg. 2020;35:1287–1294. doi: 10.1111/jocs.14596. [DOI] [PubMed] [Google Scholar]
- 8.Case fatality rate in patients with COVID-19 infection and its relationship with length of follow up. Giorgi Rossi P, Broccoli S, Angelini P. J Clin Virol. 2020;128:104415. doi: 10.1016/j.jcv.2020.104415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Travel Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comparison of pathological changes and pathogenic mechanisms caused by H1N1 influenza virus, highly pathogenic H5N1 avian influenza virus, SARS-CoV, MERS-CoV and 2019-nCoV (Article in Chinese) Liu M, Feng RE, Li Q, Zhang HK, Wang YG. Zhonghua Bing Li Xue Za Zhi. 2020;49:511–516. doi: 10.3760/cma.j.cn112151-20200301-00155. [DOI] [PubMed] [Google Scholar]
- 11.Histopathology of Middle East respiratory syndrome coronavirus (MERS-CoV) infection – clinicopathological and ultrastructural study. Alsaad KO, Hajeer AH, Al Balwi M, et al. Histopathology. 2018;72:516–524. doi: 10.1111/his.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Franks TJ, Chong PY, Chui P, et al. Hum Pathol. 2003;34:743–748. doi: 10.1016/S0046-8177(03)00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Carsana L, Sonzogni A, Nasr A, et al. Lancet Infect Dis. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Collection and submission of postmortem specimens from deceased persons with known or suspected COVID-19. [ Mar; 2022 ];https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-postmortem-specimens.html 2020
- 15.The importance of the autopsy in emerging and reemerging infectious diseases. Schwartz DA, Herman CJ. Clin Infect Dis. 1996;23:248–254. doi: 10.1093/clinids/23.2.248. [DOI] [PubMed] [Google Scholar]
- 16.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Moher D, Liberati A, Tetzlaff J, Altman DG. PLoS Med. 2009;6:0. [PMC free article] [PubMed] [Google Scholar]
- 17.Rayyan-a web and mobile app for systematic reviews. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Methodological quality and synthesis of case series and case reports. Murad MH, Sultan S, Haffar S, Bazerbachi F. BMJ Evid Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton, Canada: The GRADE Working Group; 2013. The GRADE Handbook for Grading the Quality of Evidence and Strength of Recommendations. [Google Scholar]
- 20.Postmortem examination of patients with COVID-19. Schaller T, Hirschbühl K, Burkhardt K, Braun G, Trepel M, Märkl B, Claus R. JAMA. 2020;323:2518–2520. doi: 10.1001/jama.2020.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. Rapkiewicz AV, Mai X, Carsons SE, et al. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Postmortem lung findings in a patient with asthma and coronavirus disease 2019. Konopka KE, Wilson A, Myers JL. Chest. 2020;158:0. doi: 10.1016/j.chest.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diffuse alveolar damage (DAD) resulting from coronavirus disease 2019 infection is morphologically indistinguishable from other causes of DAD. Konopka KE, Nguyen T, Jentzen JM, et al. Histopathology. 2020;77:570–578. doi: 10.1111/his.14180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.COVID-19 autopsies, Oklahoma, USA. Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. Am J Clin Pathol. 2020;153:725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Xu Z, Shi L, Wang Y, et al. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. J Thorac Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, Xiao SY. Mod Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Su H, Yang M, Wan C, et al. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Buja LM, Wolf DA, Zhao B, et al. Cardiovasc Pathol. 2020;48:107233. doi: 10.1016/j.carpath.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Bradley BT, Maioli H, Johnston R, et al. Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Wichmann D, Sperhake JP, Lütgehetmann M, et al. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pathology and pathogenesis of SARS-CoV-2 associated with fatal coronavirus disease, United States. Martines RB, Ritter JM, Matkovic E, et al. Emerg Infect Dis. 2020;26:2005–2015. doi: 10.3201/eid2609.202095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.SARS-CoV-2 infection-associated hemophagocytic lymphohistiocytosis. Prilutskiy A, Kritselis M, Shevtsov A, et al. Am J Clin Pathol. 2020;154:466–474. doi: 10.1093/ajcp/aqaa124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Zhang H, Zhou P, Wei Y, et al. Ann Intern Med. 2020;172:629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Multiscale 3-dimensional pathology findings of COVID-19 diseased lung using high-resolution cleared tissue microscopy [PREPRINT] Li G, Fox SE, Summa B, et al. bioRxiv. 2020 [Google Scholar]
- 37.Preliminary post-mortem COVID-19 evidence of endothelial injury and factor VIII hyperexpression. Cipolloni L, Sessa F, Bertozzi G, et al. Diagnostics (Basel) 2020;10:575. doi: 10.3390/diagnostics10080575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lung fibrosis: an undervalued finding in COVID-19 pathological series. Grillo F, Barisione E, Ball L, Mastracci L, Fiocca R. Lancet Infect Dis. 2021;21:0. doi: 10.1016/S1473-3099(20)30582-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pathological changes of the spleen in ten patients with coronavirus disease 2019 (COVID-19) by postmortem needle autopsy (Article in Chinese) Xu X, Chang XN, Pan HX, et al. Zhonghua Bing Li Xue Za Zhi. 2020;49:576–582. doi: 10.3760/cma.j.cn112151-20200401-00278. [DOI] [PubMed] [Google Scholar]
- 40.Pathological changes of fatal coronavirus disease 2019 (COVID-19) in the lungs: report of 10 cases by postmortem needle autopsy (Article in Chinese) Wu JH, Li X, Huang B, et al. Zhonghua Bing Li Xue Za Zhi. 2020;49:568–575. doi: 10.3760/cma.j.cn112151-20200405-00291. [DOI] [PubMed] [Google Scholar]
- 41.COVID-19 autopsy in people who died in community settings: the first series. Youd E, Moore L. J Clin Pathol. 2020;73:840–844. doi: 10.1136/jclinpath-2020-206710. [DOI] [PubMed] [Google Scholar]
- 42.Gross and histopathological pulmonary findings in a COVID-19 associated death during self-isolation. Suess C, Hausmann R. Int J Legal Med. 2020;134:1285–1290. doi: 10.1007/s00414-020-02319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Histopathological findings in a COVID-19 patient affected by ischemic gangrenous cholecystitis. Bruni A, Garofalo E, Zuccalà V, et al. World J Emerg Surg. 2020;15:43. doi: 10.1186/s13017-020-00320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Colmenero I, Santonja C, Alonso-Riaño M, et al. Br J Dermatol. 2020;183:729–737. doi: 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pathological findings of postmortem biopsies from lung, heart, and liver of 7 deceased COVID-19 patients. Beigmohammadi MT, Jahanbin B, Safaei M, et al. Int J Surg Pathol. 2021;29:135–145. doi: 10.1177/1066896920935195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Germany’s first COVID-19 deceased: a 59-year-old man presenting with diffuse alveolar damage due to SARS-CoV-2 infection. Heinrich F, Sperhake JP, Heinemann A, et al. Virchows Arch. 2020;477:335–339. doi: 10.1007/s00428-020-02872-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. Wang C, Xie J, Zhao L, et al. EBioMedicine. 2020;57:102833. doi: 10.1016/j.ebiom.2020.102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Acta Neuropathol. 2020;140:1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.A pathological report of three COVID-19 cases by minimal invasive autopsies (Article in Chinese) Yao XH, Li TY, He ZC, et al. Zhonghua Bing Li Xue Za Zhi. 2020;49:411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 50.Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Menter T, Haslbauer JD, Nienhold R, et al. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Edler C, Schröder AS, Aepfelbacher M, et al. Int J Legal Med. 2020;134:1275–1284. doi: 10.1007/s00414-020-02317-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Remmelink M, De Mendonça R, D’Haene N, et al. Crit Care. 2020;24:495. doi: 10.1186/s13054-020-03218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Lax SF, Skok K, Zechner P, et al. Ann Intern Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Magro C, Mulvey JJ, Berlin D, et al. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pathophysiology of SARS-CoV-2: the Mount Sinai COVID-19 autopsy experience. Bryce C, Grimes Z, Pujadas E, et al. Mod Pathol. 2021;34:1456–1467. doi: 10.1038/s41379-021-00793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Evidence for systematic autopsies in COVID-19 positive deceased: case report of the first German investigated COVID-19 death. Fitzek A, Sperhake J, Edler C, et al. Rechtsmedizin (Berl) 2020;30:184–189. doi: 10.1007/s00194-020-00401-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clinicopathologic and immunohistochemical findings from autopsy of patient with COVID-19, Japan. Adachi T, Chong JM, Nakajima N, et al. Emerg Infect Dis. 2020;26:353. doi: 10.3201/eid2609.201353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Late histopathologic characteristics of critically ill COVID-19 patients: different phenotypes without evidence of invasive aspergillosis, a case series. Flikweert AW, Grootenboers MJ, Yick DC, du Mée AW, van der Meer NJ, Rettig TC, Kant MK. J Crit Care. 2020;59:149–155. doi: 10.1016/j.jcrc.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. Ackermann M, Verleden SE, Kuehnel M, et al. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.In situ detection of SARS-CoV-2 in lungs and airways of patients with COVID-19. Schaefer IM, Padera RF, Solomon IH, Kanjilal S, Hammer MM, Hornick JL, Sholl LM. Mod Pathol. 2020;33:2104–2114. doi: 10.1038/s41379-020-0595-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.The evolution of pulmonary pathology in fatal COVID-19 disease: an autopsy study with clinical correlation. Bösmüller H, Traxler S, Bitzer M, et al. Virchows Arch. 2020;477:349–357. doi: 10.1007/s00428-020-02881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Sonzogni A, Previtali G, Seghezzi M, et al. Liver Int. 2020;40:2110–2116. doi: 10.1111/liv.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. Wang Y, Liu S, Liu H, et al. J Hepatol. 2020;73:807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Complete post-mortem data in a fatal case of COVID-19: clinical, radiological and pathological correlations. Ducloyer M, Gaborit B, Toquet C, et al. Int J Legal Med. 2020;134:2209–2214. doi: 10.1007/s00414-020-02390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neuropathologic features of four autopsied COVID-19 patients. Kantonen J, Mahzabin S, Mäyränpää MI, et al. Brain Pathol. 2020;30:1012–1016. doi: 10.1111/bpa.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Histopathological findings in the advanced natural evolution of the SARS-CoV-2 infection. Cîrstea AE, Buzulică RL, Pirici D, et al. Rom J Morphol Embryol. 2020;61:209–218. doi: 10.47162/RJME.61.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fatal pulmonary fibrosis: a post-COVID-19 autopsy case (Epub ahead of print) Schwensen HF, Borreschmidt LK, Storgaard M, Redsted S, Christensen S, Madsen LB. J Clin Pathol. 2020 doi: 10.1136/jclinpath-2020-206879. [DOI] [PubMed] [Google Scholar]
- 68.Postmortem kidney pathology findings in patients with COVID-19. Santoriello D, Khairallah P, Bomback AS, et al. J Am Soc Nephrol. 2020;31:2158–2167. doi: 10.1681/ASN.2020050744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.No autopsies on COVID-19 deaths: a missed opportunity and the lockdown of science. Salerno M, Sessa F, Piscopo A, et al. J Clin Med. 2020;9:1472. doi: 10.3390/jcm9051472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.The role of forensic pathologists in coronavirus disease 2019 infection: the importance of an interdisciplinary research. Barranco R, Ventura F. Med Sci Law. 2020;60:237–238. doi: 10.1177/0025802420927825. [DOI] [PubMed] [Google Scholar]
- 71.Management of the corpse with suspect, probable or confirmed COVID-19 respiratory infection – Italian interim recommendations for personnel potentially exposed to material from corpses, including body fluids, in morgue structures and during autopsy practice. Fineschi V, Aprile A, Aquila I, et al. Pathologica. 2020;112:64–77. doi: 10.32074/1591-951X-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Review: the safe handling of a corpse (suspected) with COVID-19. Dijkhuizen LG, Gelderman HT, Duijst WL. J Forensic Leg Med. 2020;73:101999. doi: 10.1016/j.jflm.2020.101999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of Middle East respiratory syndrome coronavirus infection in the United Arab Emirates, April 2014. Ng DL, Al Hosani F, Keating MK, et al. Am J Pathol. 2016;186:652–658. doi: 10.1016/j.ajpath.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lung pathology of fatal severe acute respiratory syndrome. Nicholls JM, Poon LL, Lee KC, et al. Lancet. 2003;361:1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Autopsy findings in eight patients with fatal H1N1 influenza. Harms PW, Schmidt LA, Smith LB, et al. Am J Clin Pathol. 2010;134:27–35. doi: 10.1309/AJCP35KOZSAVNQZW. [DOI] [PubMed] [Google Scholar]
- 76.Interesting post-mortem findings in a H1N1 influenza-positive pneumonia patient. Balraam KV, Sidhu A, Srinivas V. Autops Case Rep. 2019;9:0. doi: 10.4322/acr.2018.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Histopathological and immunohistochemical findings of 20 autopsy cases with 2009 H1N1 virus infection. Nakajima N, Sato Y, Katano H, et al. Mod Pathol. 2012;25:1–13. doi: 10.1038/modpathol.2011.125. [DOI] [PubMed] [Google Scholar]
- 78.Histopathological autoptic findings in 8 patients with pandemic influenza A (H1N1) pneumonia. Skálová H, Povýšil C, Hofmanová J, Goldová B, Jakša R, Jandová K, Galko J. https://pubmed.ncbi.nlm.nih.gov/23057432/ Cesk Patol. 2012;48:161–164. [PubMed] [Google Scholar]
- 79.Systematic review of COVID-19 related myocarditis: insights on management and outcome. Sawalha K, Abozenah M, Kadado AJ, et al. Cardiovasc Revasc Med. 2021;23:107–113. doi: 10.1016/j.carrev.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.A clinicopathological study of three cases of severe acute respiratory syndrome (SARS) Lang ZW, Zhang LJ, Zhang SJ, et al. Pathology. 2003;35:526–531. doi: 10.1080/00313020310001619118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: challenges in determining a SARS diagnosis. Chong PY, Chui P, Ling AE, et al. Arch Pathol Lab Med. 2004;128:195–204. doi: 10.5858/2004-128-195-AODDTS. [DOI] [PubMed] [Google Scholar]
- 82.Multiple organ infection and the pathogenesis of SARS. Gu J, Gong E, Zhang B, et al. J Exp Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Acute renal failure in SARS patients: more than rhabdomyolysis. Wu VC, Hsueh PR, Lin WC, Huang JW, Tsai HB, Chen YM, Wu KD. Nephrol Dial Transplant. 2004;19:3180–3182. doi: 10.1093/ndt/gfh436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Chau TN, Lee KC, Yao H, et al. Hepatology. 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. Wong RS, Wu A, To KF, et al. BMJ. 2003;326:1358–1362. doi: 10.1136/bmj.326.7403.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cerebrospinal fluid findings in COVID-19 patients with neurological symptoms. Neumann B, Schmidbauer ML, Dimitriadis K, et al. J Neurol Sci. 2020;418:117090. doi: 10.1016/j.jns.2020.117090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Xu J, Zhong S, Liu J, et al. Clin Infect Dis. 2005;41:1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]